Find out more about the efficacy of SKYRIZI
Explore the durability of response with SKYRIZI through clinical trials and long-term data in psoriasis and psoriatic arthritis. Start by selecting a study from the menu below.
LONG-TERM DATA
HEAD-TO-HEAD TRIALS
SKYRIZI IN PsA
HEAD-TO-HEAD TRIALS: SKYRIZI vs Adalimumab (IMMvent)
Superior efficacy vs adalimumab in psoriasis1
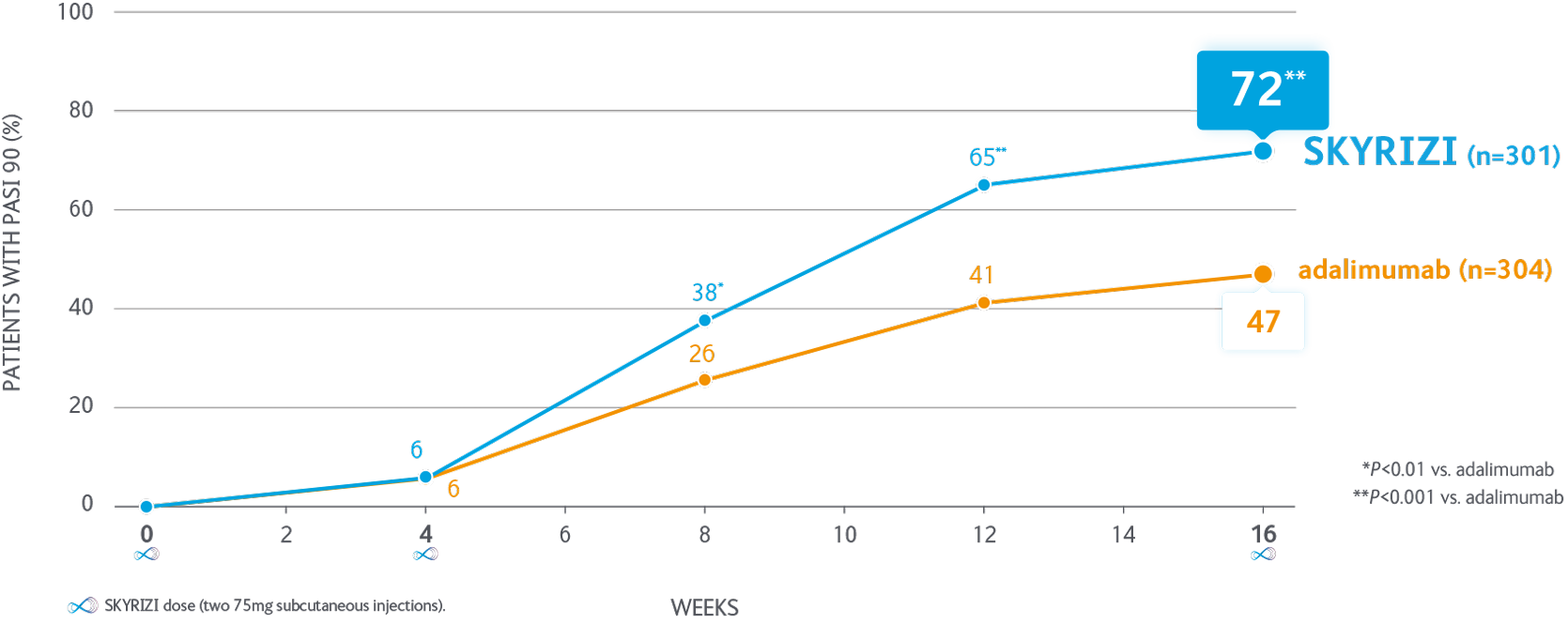
Significantly more patients achieve PASI 90 with SKYRIZI vs adalimumab at 16 weeks1
Proportion of patients achieving PASI 90 through to Week 16 in the IMMvent pivotal trial
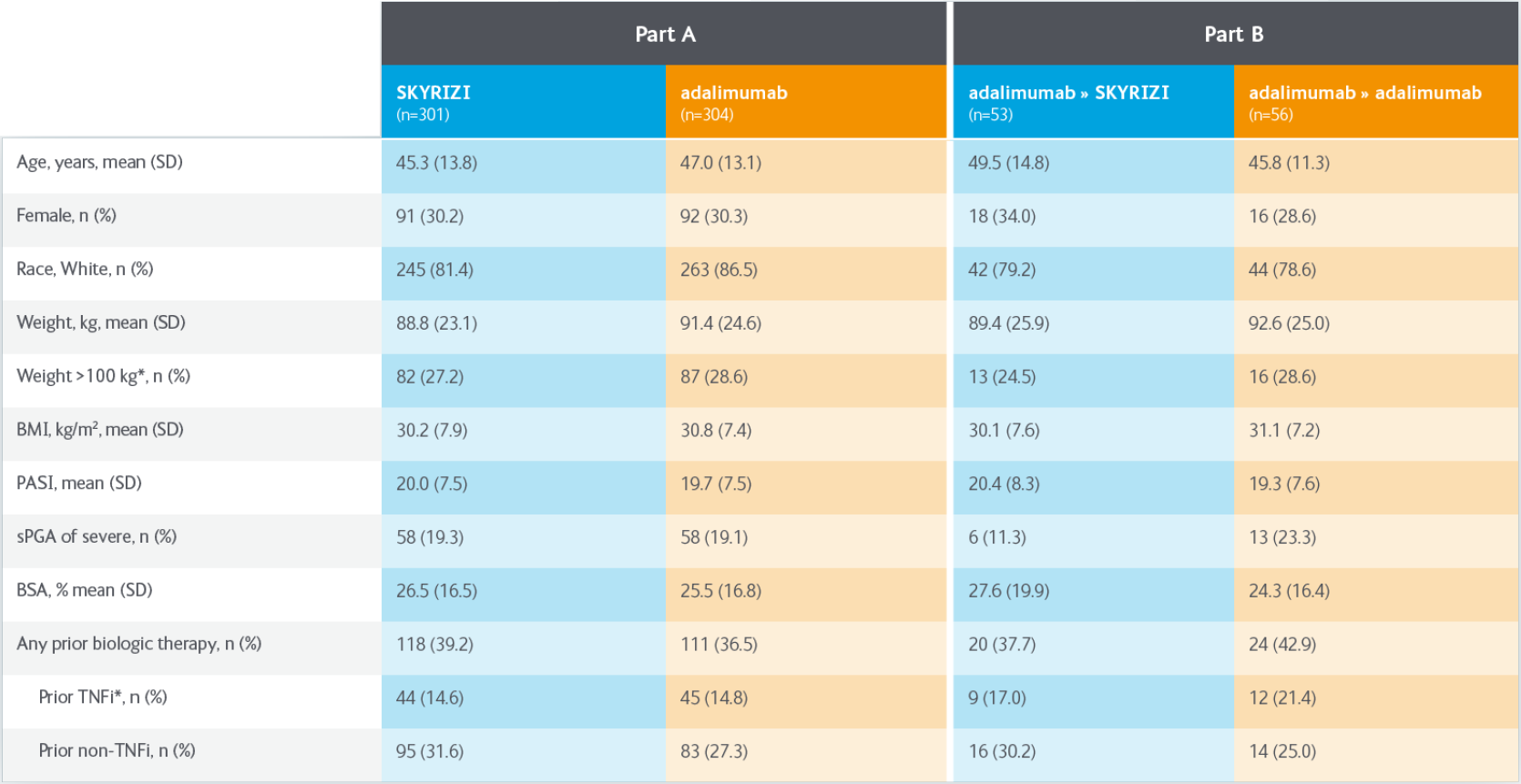
IMMvent was a phase III, randomised, double-blind, active-controlled study evaluating the efficacy and safety of SKYRIZI compared with adalimumab in adult patients with moderate to severe plaque psoriasis. SKYRIZI n=301. adalimumab n=304.
Co-primary endpoints for Part A - PASI 90 & sPGA 0/1 at Week 16. SKYRIZI vs adalimumab were met P<0.001.
Intention to treat population.
PASI, Psoriasis Area and Severity Index; sPGA, static Physician’s Global Assessment.
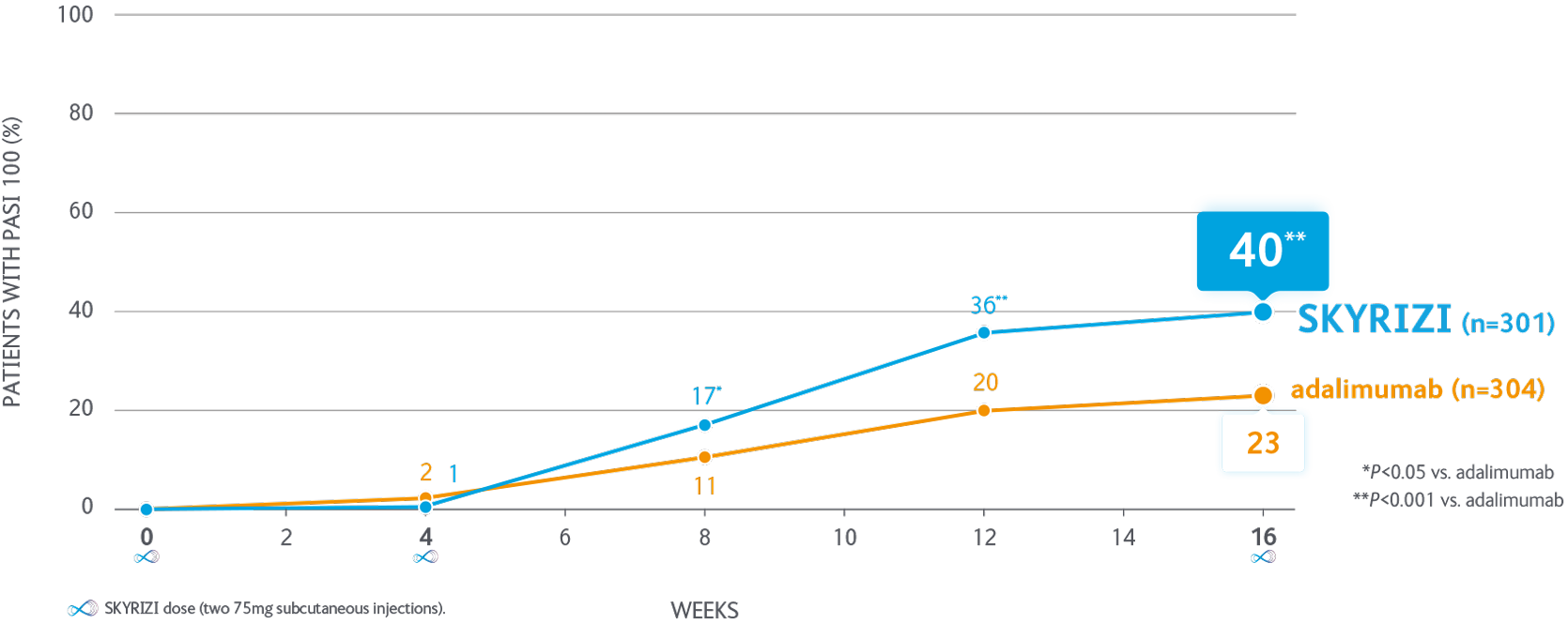
Significantly more patients achieve PASI 100 with SKYRIZI vs adalimumab at 16 weeks1
Proportion of patients achieving PASI 100 through to Week 16 in the IMMvent pivotal trial
IMMvent was a phase III, randomised, double-bllnd. active-controlled study evaluating the efficacy and safety of SKYRIZI compared with adalimumab in adult patients with moderate to severe plaque psoriasis. SKYRIZI n=301, adalimumab n=304.
Co-primary endpoints for Part A - PASI 90 & sPGA 0/1 at Week 16. SKYRIZI vs adalimumab were met P<0.001.
Intention to treat population.
PASI, Psoriasis Area and Severity Index; sPGA, static Physician’s Global Assessment.
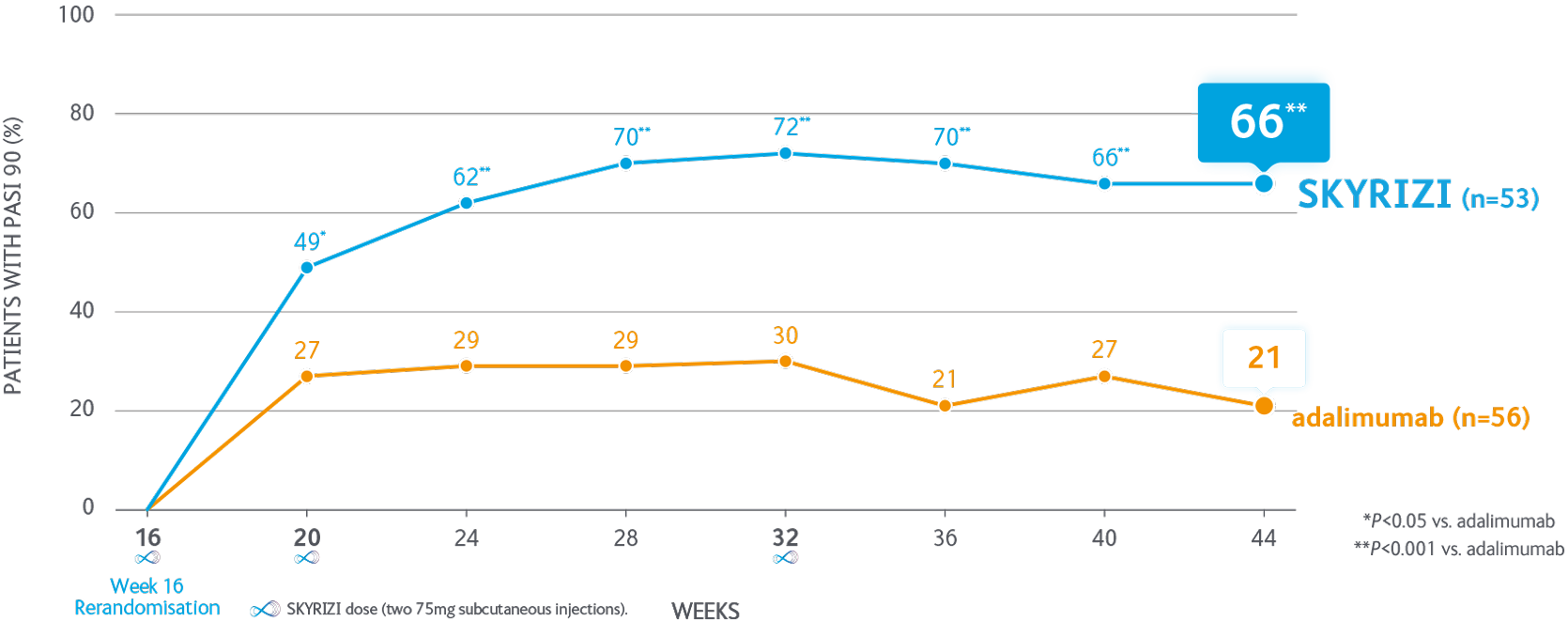
SKYRIZI significantly improved PASI 90 in inadequate or partial responders after switching from adalimumab1
66% of SKYRIZI patients achieved PASI 90 at Week 44 vs 21% continuing on adalimumab1
Inadequate or partial response In the IMMvent study was defined as >PASI 50 to <PASI 90 at Week 16 in patients receiving adalimumab.
Not depicted: IMMvent Part A (baseline to Week 16). SKYRIZI (n=301): adalimumab (n=304). PASI 90 at Week 16: SKYRIZI 72.4%; adalimumab 47.4% (P<0.001), inadequate or partial responders only (≤PASI 50 to <PASI 90).
Co-primary endpoints for Part A - PASI 90 & sPGA 0/1 at Week 16. SKYRIZI vs adalimumab were met P<0.001.
aPrimary endpoint for Part B, PASI 90 at Week 44 in re-randomised patients.
PASI, Psoriasis Area and Severity Index; sPGA, static Physician’s Global Assessment.
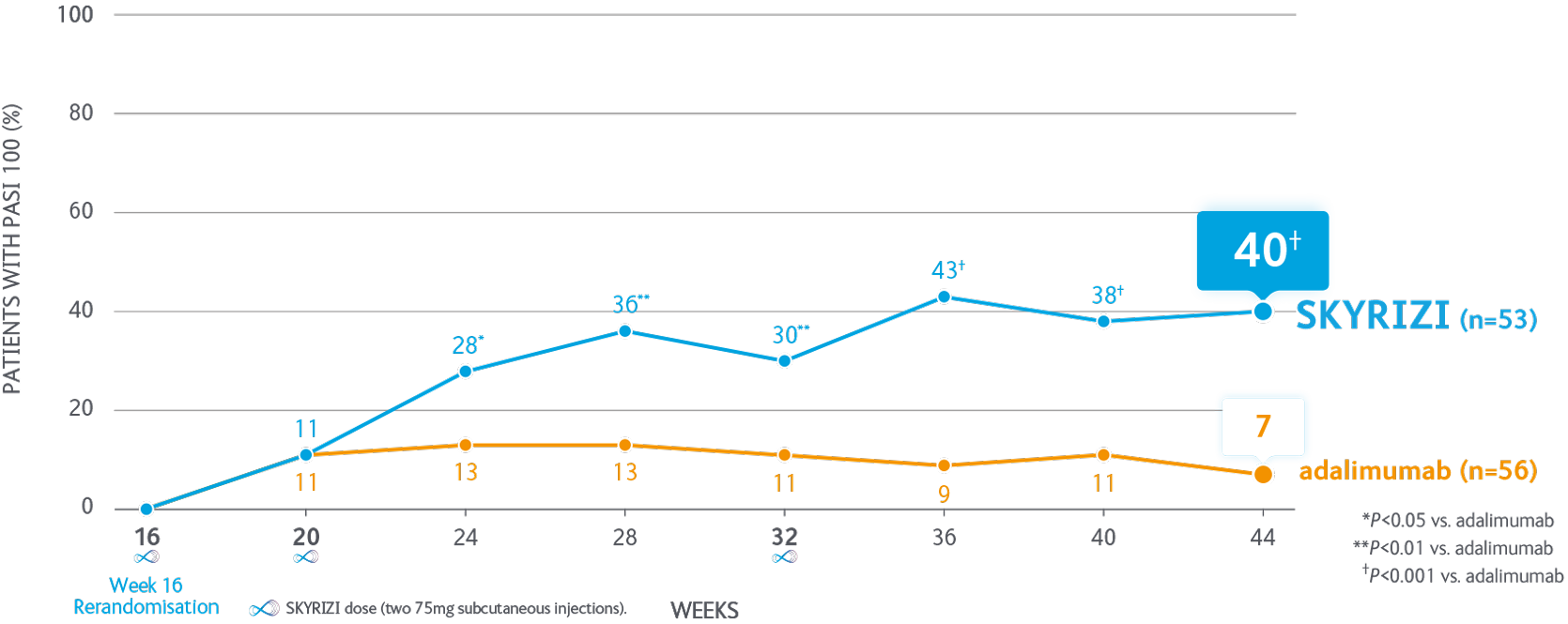
More than 60% of SKYRIZI patients maintained PASI 100 through to 2.5 years1
40% of SKYRIZI patients achieved PASI 100 at Week 44 vs 7% continuing on adalimumab1
Inadequate or partial response In the IMMvent study was defined as ≥PASI 50 to <PASI 90 at Week 16 in patients receiving adalimumab. Not depicted: IMMvent Part A (baseline to Week 16). SKYRIZI (n=301); adalimumab (n=304). PASI 90 at Week 16: SKYRIZI 72.4%; adalimumab 47.4% (P<0.001). Inadequate or partial responders only (≥PASI 50 to <PASI 90) were re-randomised to Part B. Primary endpoint for Part B was PASI 90 at Week 44 with SKYRIZI vs adalimumab among those with PASI 50-<PASI 90 after 16 weeks of adalimumab treatment. Part B endpoint was met (P<0.001).
Co-primary endpoints for Part A - PASI 90 & sPGA 0/1 at Week 16. SKYRIZI vs adalimumab were met P<0.001.
aRanked secondary endpoint.
PASI, Psoriasis Area and Severity Index; sPGA, static Physician’s Global Assessment.
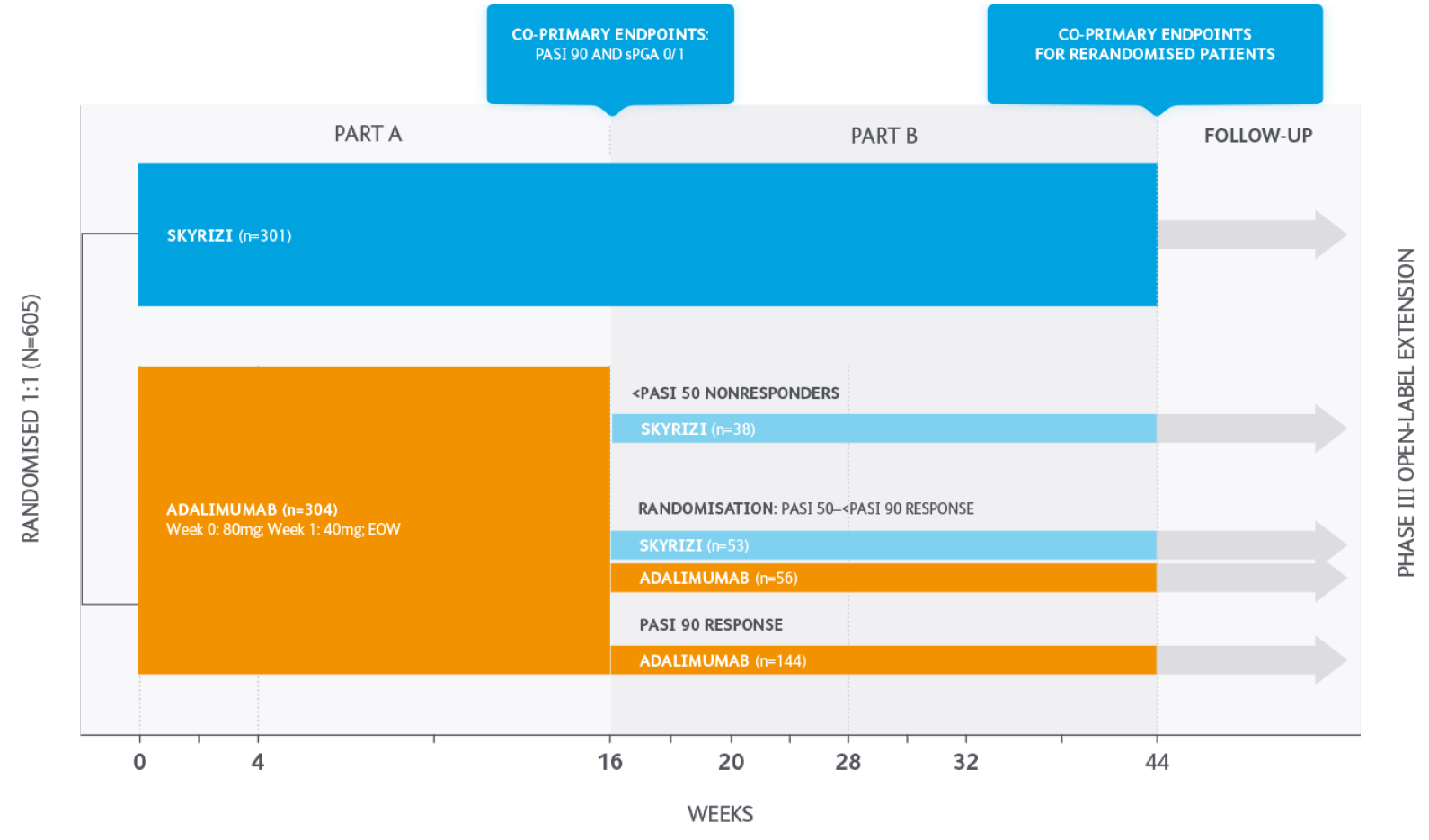
Efficacy and safety of SKYRIZI compared with adalimumab in patients with moderate to severe plaque psoriasis
Part A (baseline to Week 16): Patients received either SKYRIZI 150mg or adalimumab (80mg at baseline, 40mg at Week 1, and then EOW).
Part B (Weeks 16 to 52): SKYRIZI patients continued on treatment. Adalimumab group, dependent on PASI response:
- PASI <50 switched to SKYRIZI
- PASI 50 <PASI 90 (re-randomised to either SKYRIZI or adalimumab)
- PASI 90 continued with adalimumab
EOW, every other week; PASI, Psoriasis Area Severity Index; sPGA, static Physicians’ Global Assessment.
Co-primary endpoints:
Co-primary endpoints for Part A were PASI 90 and sPGA 0/1 at week 16 with SKYRIZI vs adalimumab.
Primary endpoint for Part B was PASI 90 at Week 44 with SKYRIZI vs adalimumab among those with PASI 50 — PASI 90 after 16 weeks of adalimumab treatment.
Co-primary and primary endpoints were met for both Part A and Part B (P<0.0001).
Ranked secondary endpoints:
- PASI 75 at Week 16 with SKYRIZI vs adalimumab
- PASI 100 at Weeks 16 and 44 with SKYRIZI vs adalimumab.
All ranked endpoints were met (P<0.001)
PASI, Psoriasis Area Severity Index; sPGA, static Physicians’ Global Assessment.
A similar safety profile to adalimumab⁵
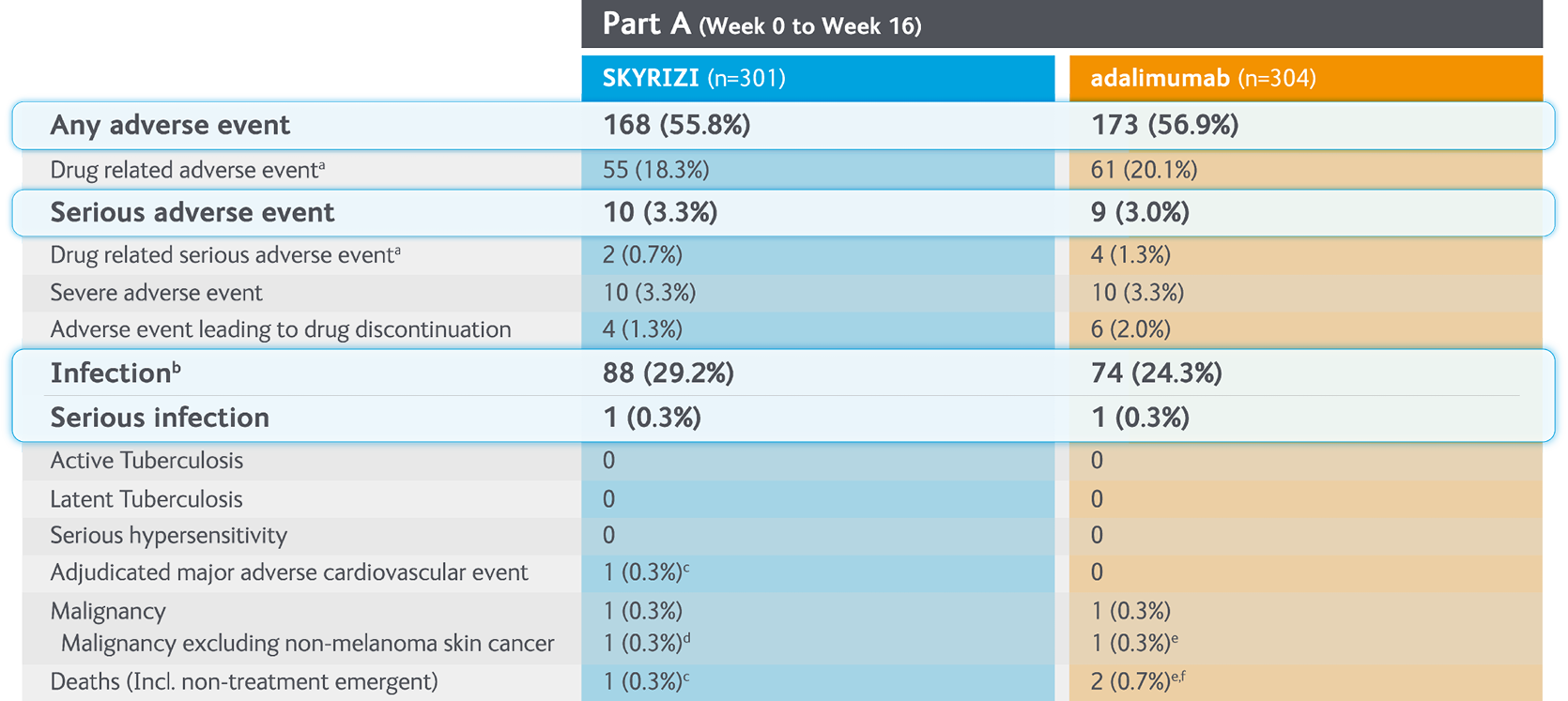
Summary of treatment-emergent adverse events with SKYRIZI vs adalimumab in Part A (Weeks 0-16) of the IMMvent Phase III clinical trial5
Randomised controlled trial, intention to treat population (Part A).
aInvestigator assessed AE as possibly related to study drug.
bThe most frequently reported infectious AE were viral upper respiratory tract infection and upper respiratory tract infection.
cOne patient with acute myocardial infarction on study day 73 (event was not considered to be study drug related by investigator).
dOne patient diagnosed with invasive lobular breast carcinoma on study day 63 following routine mammogram (event was not considered to be study drug related by investigator).
eOne patient with stage IV gall bladder cancer.
fOne patient with cholelithiasis, underwent gall bladder surgery, developed cardiopulmonary arrest and died due to abdominal abscess, sepsis, and gastric perforation (events were not considered to be study drug related by investigator).
AE, adverse event; MACE, major adverse cardiovascular events; NMSC, non-melanoma skin cancer.
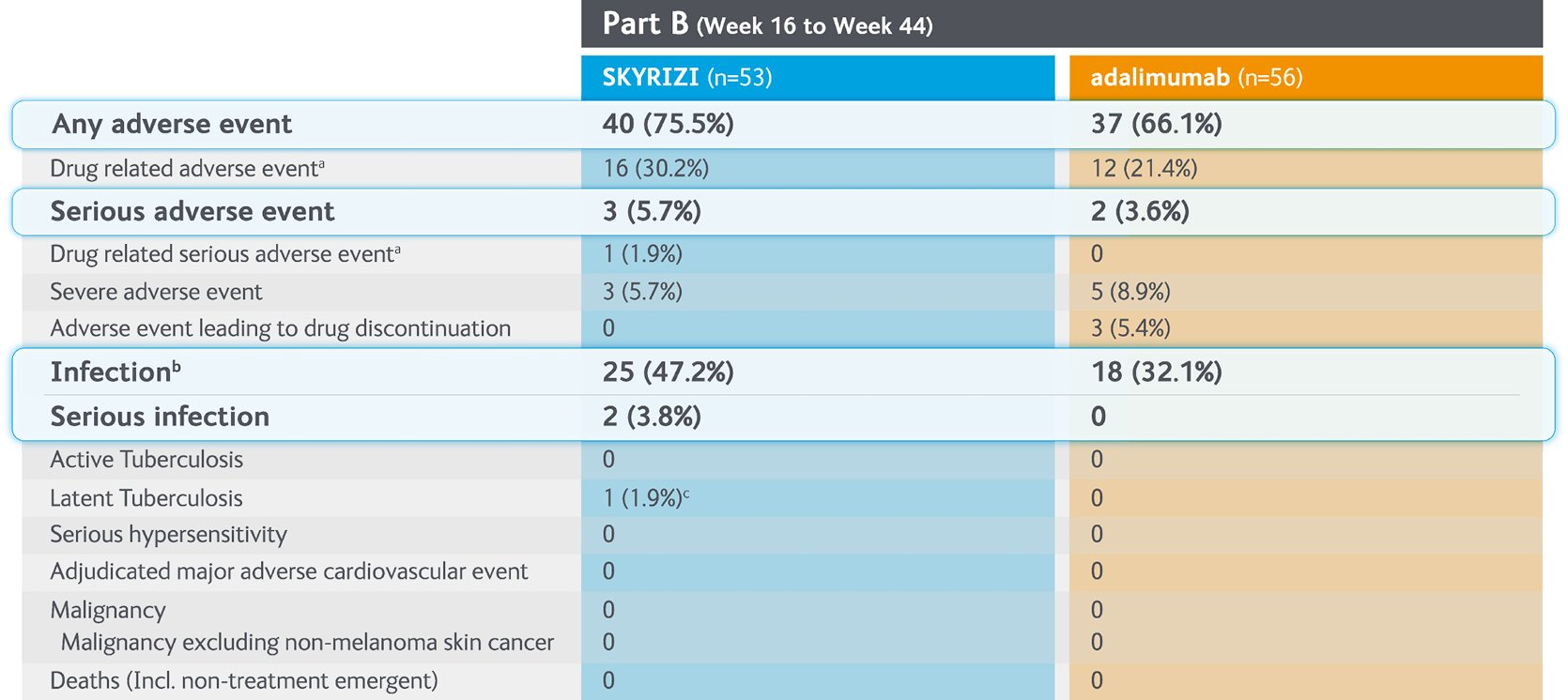
Summary of treatment-emergent adverse events with SKYRIZI vs. adalimumab in Part B (Weeks 16-44) of the IMMvent Phase III clinical trial7
Randomised controlled trial. lntention to population (Part B). Patients previously treated with adailmumab. randomised to adallrnumab or SKYRIZI at Week 16.
aInvestigator assessed AE as possibly related to study drug.
bThe most frequently reported Infectious AE were viral upper respiratory tract Infection and upper respiratory tract Infection.
cOne patient with latent tuberculosis. positive TB test during the study.
AE, adverse event; MACE, major adverse cardiovascular events; NMSC, non-melanoma skin cancer.
Featured content
UK-RISN-240174. Date of preparation May 2024.
References
- Reich K, et al. Lancet 2019; 394: 576-586.
- Gordon KB, et al. Lancet 2018; 392: 650-661.
- Warren RB, et al. Risankizumab vs Secukinumab in Patients with Moderate-to-Severe Plaque Psoriasis: A Phase 3 Trial, Presented at AAD 2020.
- SKYRIZI: Summary of Product Characteristics.
- Reich K, et al. Poster 1813, Efficacy and Safety of Risankizumab Compared with Adalimumab in Patients with Moderate-to-Severe Plaque Psoriasis: Results from the Phase 3 IMMvent Trial. 2018.
UK-RISN-240137. Date of preparation: May 2024.