This promotional material is intended for UK Healthcare Professionals only.
BOTOX® (botulinum toxin type A) Prescribing Information and adverse event reporting information can be found below.
Understanding and addressing the unmet need in urology: Neurogenic Detrusor Overactivity (NDO)
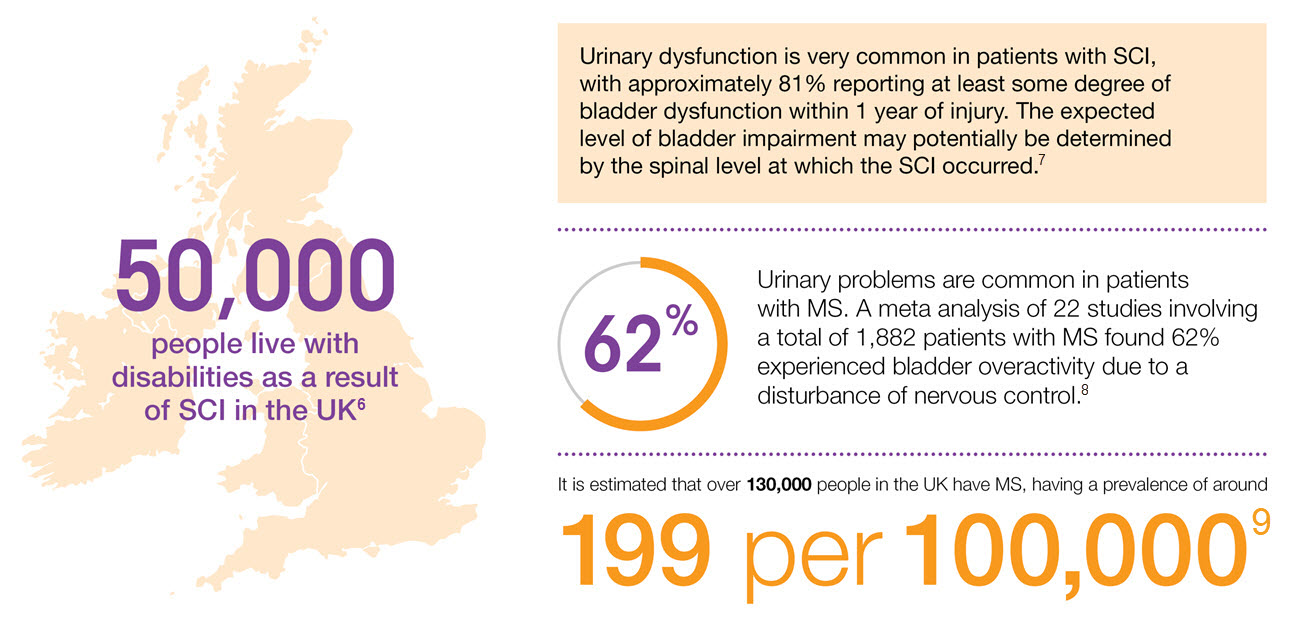
Prevalence of NDO
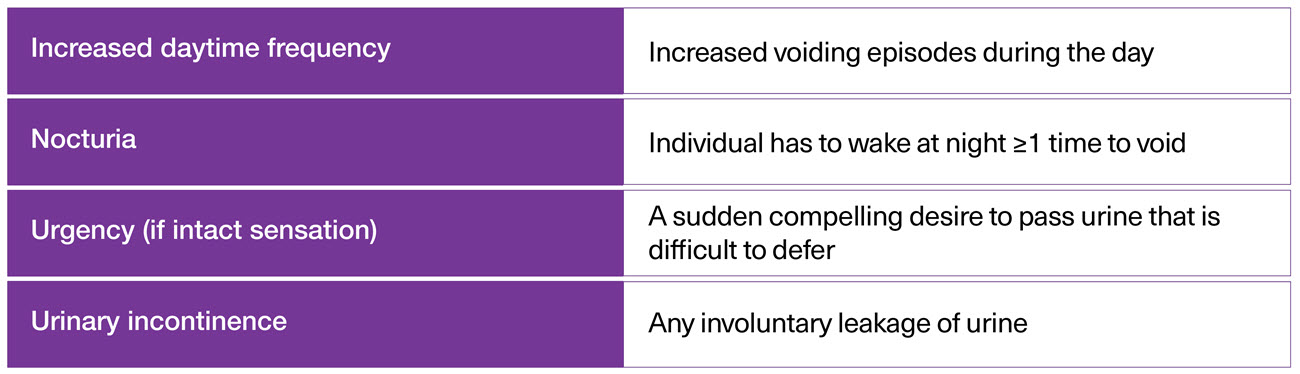
NDO is a complex, multi-symptom syndrome that can be difficult to treat, requiring appropriate effective management - including treatment or referrals to improve patient outcomes
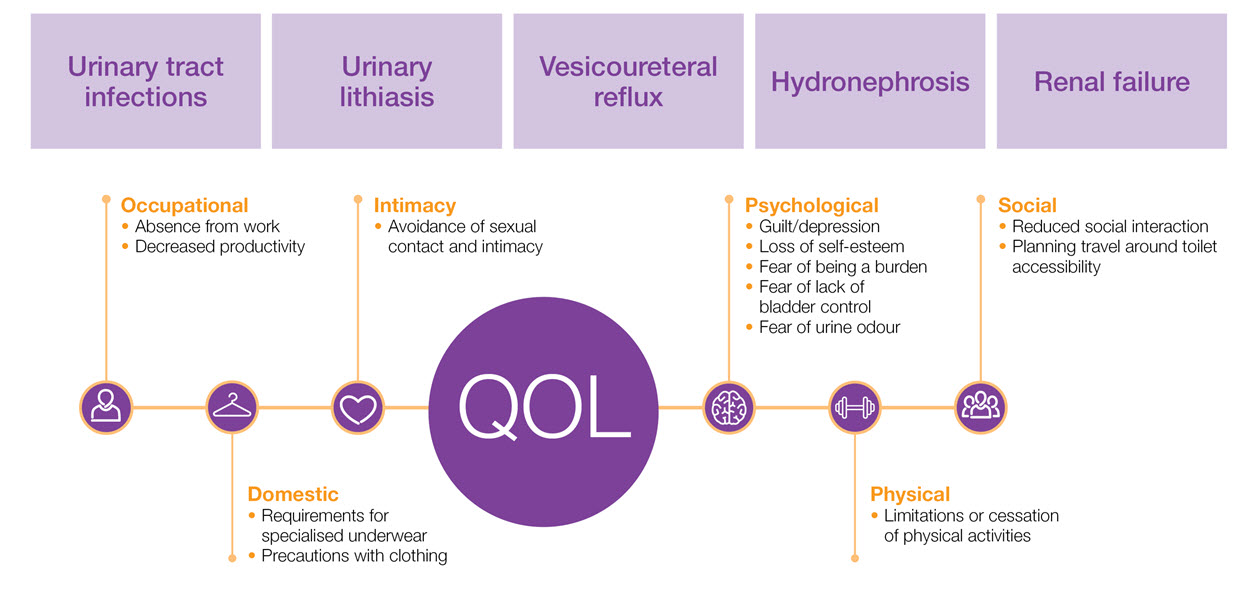
The aetiology of NDO10,11
MS, multiple sclerosis; NDO, neurogenic detrusor overactivity; QoL: quality of life; SCI, spinal cord injury.
Please refer to the BOTOX® Summary of Product Characteristics for further information on adverse events, contraindications and special warnings and precautions for use. The BOTOX® Summary of Product Characteristics can be found here
By clicking the link above you will leave the AbbVie Pro website and be taken to the eMC PI portal website.
Adverse events should be reported. Reporting forms and information can be found at https://yellowcard.mhra.gov.uk/
Adverse events should also be reported to AbbVie on GBPV@abbvie.com
Date of preparation: June 2025. UK-BUO-250049.