GOAL SETTING IN POST-STROKE SPASTICITY (PSS):
Why is it important when treating patients with Botulinum Toxin Type A?
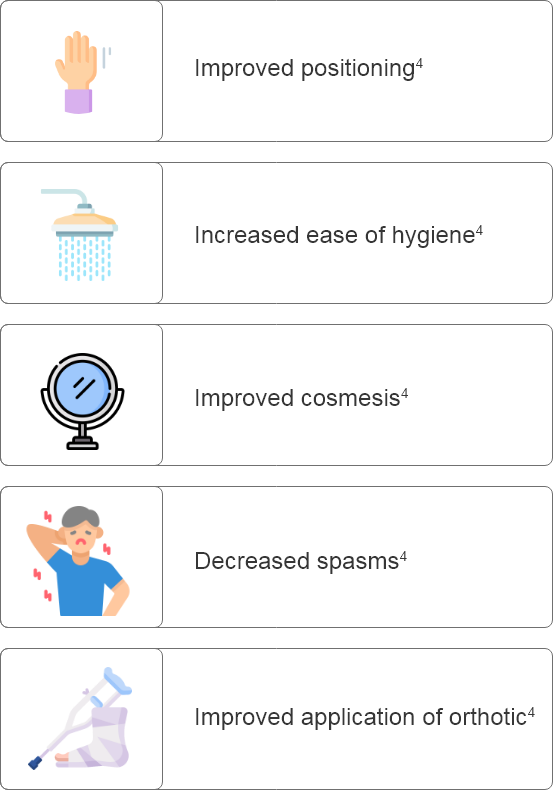
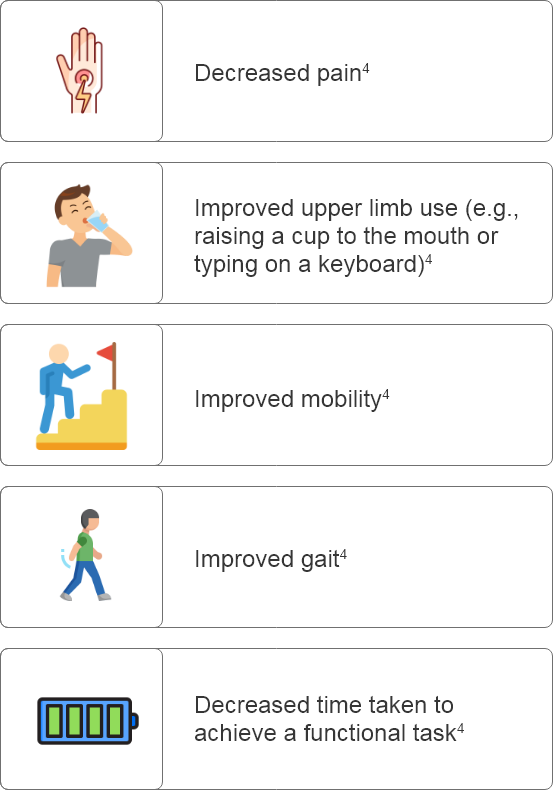
Why set goals in patients with PSS?
GOAL SETTING IN POST-STROKE SPASTICITY (PSS):
Why is it important when treating patients with Botulinum Toxin Type A?
What are some considerations for setting goals prior to Botulinum Toxin Type A treatment?
How can goals be assessed effectively after Botulinum Toxin Type A administration?
Re-evaluation period to be updated during local approval to reflect local guidelines.
Adapted from Turner-Stokes L. et al. 2010.7
*At baseline, a score of -1 is given to allow for deterioration.
If no clinically plausible worse outcome is possible, then a score of -2 is given.7
This material has been funded by Allergan, an AbbVie Company.
Botulinum Toxin Type A is indicated for the symptomatic treatment of:9
- Focal spasticity of the wrist and hand in adult post-stroke patients.
- Focal spasticity of the ankle and foot in adult post-stroke patients.
Adverse events should be reported to your local regulatory authority and AbbVie or Allergan, an AbbVie Company.
- Although used to assess spasticity in both adult and paediatric cases, the reliability for assessing paediatric spasticity is debated12-15
- Measures increase in muscle tone11
- Easily applied in a clinical environment11
- Does not require measuring equipment11
- Cannot distinguish spasticity from other tonus disorders11
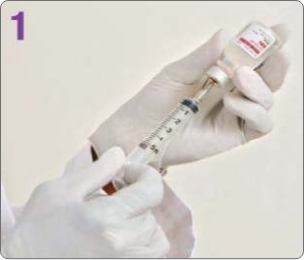
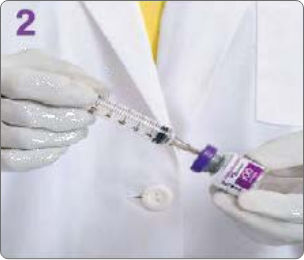
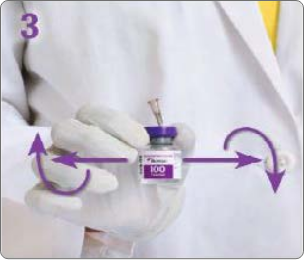
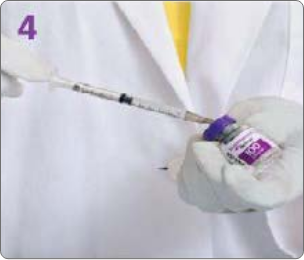
BOTULINUM TOXIN RECONSTITUTION
Insert the needle straight into the vial, then tilt the vial at a 45◦ angle. Slowly inject the saline into the Botulinum Toxin Type A neurotoxin vial. A vacuum is present in the vial, which demonstrates that the sterility of the vial is intact. Do not use the vial if the vacuum does not pull the saline into the vial.
*The following muscles contribute to this posture but are not shown as they are more readily identified in the anterior compartment of the forearm, which is not visible here: Pronator quadratus, flexor carpi radialis, flexor carpi ulnaris, flexor digitorum profundus, flexor digitorum superficialis, flexor pollicis longus, flexor pollicis brevis, opponens pollicis, interossei, and lumbricals.
**For anatomical reference only.
†The first dorsal interosseous was made transparent to improve the visibility of the lumbricals.
AbbVie Limited. BOTOXTM Summary of Product Characteristics.
References
1. Ashford, S., Turner-Stokes, L.F., Allison, R., Duke, L., Moore, P., Bavikatte, G., Kirker, S., Ward, A.B. and Bilton, D., (2018), March. Spasticity in adults: management using botulinum toxin. National guidelines. In Royal College of Physicians.
2. Stroke Foundation. (2014). Goal Setting.. Last Updated: September 12, 2014. Available at: https://strokefoundation.org.au/media-centre/stroke-stories/goal-setting [Accessed 1 August 2023].
3. Clarke, D.J. and Forster, A. (2015). Improving post-stroke recovery: the role of the multidisciplinary health care team. Journal of multidisciplinary healthcare, pp.433-442.
4. King’s College London UK. (2013). The GAS-eous Tool Goal Attainment Scaling – Evaluation of Outcome for Upper-limb Spasticity.. Last Updated: 30 December 2013. Available at: https://www.kcl.ac.uk/nmpc/assets/rehab/tools-gaseous-gaseoustool. pdf [Accessed 1 August 2023].
5. Stevens, A., Köke, A., van der Weijden, T. and Beurskens, A. (2017). Ready for goal setting? Process evaluation of a patient-specific goal-setting method in physiotherapy. BMC health services research, 17(1), pp.1-10.
6. Baricich, A., Wein, T., Cinone, N., Bertoni, M., Picelli, A., Chisari, C., Molteni, F. and Santamato, A., 2021. BoNT-A for post-stroke spasticity: guidance on unmet clinical needs from a delphi panel approach. Toxins, 13(4), p.236.
7. Turner-Stokes, L., Baguley, I.J., De Graaff, S., Katrak, P., Davies, L., McCrory, P. and Hughes, A. (2010). Goal attainment scaling in the evaluation of treatment of upper limb spasticity with botulinum toxin: a secondary analysis from a double-blind placebo-controlled randomized clinical trial. Journal of Rehabilitation Medicine, 42(1), pp.81-89.
8. Turner-Stokes, L. (2009). Goal attainment scaling (GAS) in rehabilitation: a practical guide. Clinical rehabilitation, 23(4), pp.362-370.
9. Allergan Ltd. BOTOX® Summary of Product Characteristics. January 2021 [Accessed 1 August 2023].
10. Meseguer-Henarejos, A.B., Sánchez-Meca, J., López-Pina, J.A. and Carles-Hernández, R. (2017). Inter-and intra-rater reliability of the Modified Ashworth Scale: a systematic review and meta-analysis. European journal of physical and rehabilitation medicine, 54(4), pp.576-590.
11. Balci, B.P. (2018). Spasticity measurement. Archives of Neuropsychiatry, 55(Suppl1), p.S49.
12. Mutlu, A., Livanelioglu, A. and Gunel, M.K. (2008). Reliability of Ashworth and Modified Ashworth scales in children with spastic cerebral palsy. BMC musculoskeletal disorders, 9(1), pp.1-8.
13. Numanoglu, A. and GUNEL, M. (2012). Intraobserver reliability of modified Ashworth scale and modified Tardieu scale in the assessment of spasticity in children with cerebral palsy. Acta orthopaedica et traumatologica turcica, 46(3), pp.196-200.
14. Fosang, A.L., Galea, M.P., McCoy, A.T., Reddihough, D.S. and Story, I. (2003). Measures of muscle and joint performance in the lower limb of children with cerebral palsy. Developmental medicine and child neurology, 45(10), pp.664-670.
15. Yam, W.K.L. and Leung, M.S.M. (2006). Interrater reliability of Modified Ashworth Scale and Modified Tardieu Scale in children with spastic cerebral palsy. Journal of child neurology, 21(12), pp.1031-1035.
BONT-BH-00006-MC