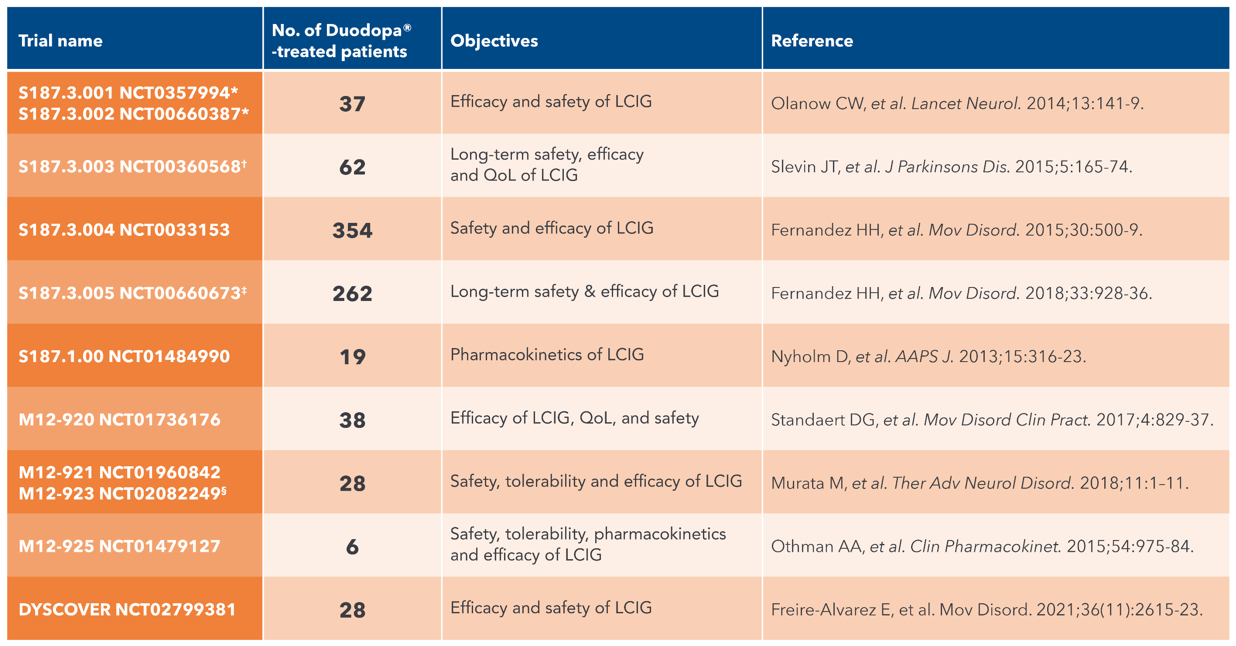
Duodopa®: Extensive world-wide experience1-3
1. Data on File. AbbVie, Inc., ABVRRTl72052.
2. Data on File. AbbVie, Inc.
3. https://www.ema.europa.eu/en/medicines/human/orphan-designations/eu301035 (Accessed November 2022).
4. Duodopa® Summary of Product Characteristics, available on www.medicines.ie.
5. Boyd JT, et al. Clin Park Relat Disord. 2020;2:25-34.
6. Valldeoriola F, et al. Mov Disord. 2018; 33 (suppl.2).
7. Fasano A, et al. Mov Disord. 2021; https://doi.org/10.1002/mds.28596.
8. Palhagen SE, et al. Parkinsonism Relat Disord. 2016;29:17-23.
9. Aldred J, et al. Neurodegener Dis Manag. 2020;10:309-23.
10. Valldeoriola F, et al. Neurodegener Dis Manag. 2016;6:289-98.
11. Antonini A, et al. Parkinsonism Relat Disord. 2017;45: 13-20.
12. Lopiano L, et al. J Neural. 2019;266:2164-76.
13. Kruger R, et al. Adv Ther. 2017;34:1741-52.
14. Aldred J, et al. Mov Disord. 2020;35 (suppl. 1 ).
15. Tessitore A, et al. J Neural. 2018;265:1124-37.
16. Antonini A, et al. J Neural Transm. 2020;127:881-91.
17. Olanow CW, et al. Lancet Neurol. 2014;13:141-9.
18. Slevin JT, et al. J Parkinsons Dis. 2015;5:165-74.
19. Fernandez HH, et al. Mov Disord. 2015;30:500-9.
20. Fernandez HH, et al. Mov Disord. 2018;33:928-36.
21. Nyholm D, et al. AAPS J. 2013; 15:316-23.
22. Standaert DG, et al. Mov Disord Clin Pract. 2017;4:829-37.
23. Murata M, et al. Ther Adv Neurol Disord. 2018; 11: 1-11.
24. Othman AA, et al. Clin Pharmacokinet. 2015;54:975-84.
25. Freire-Alvarez E, et al. Mov Disord. 2021 ;36(11 ):2615-23.
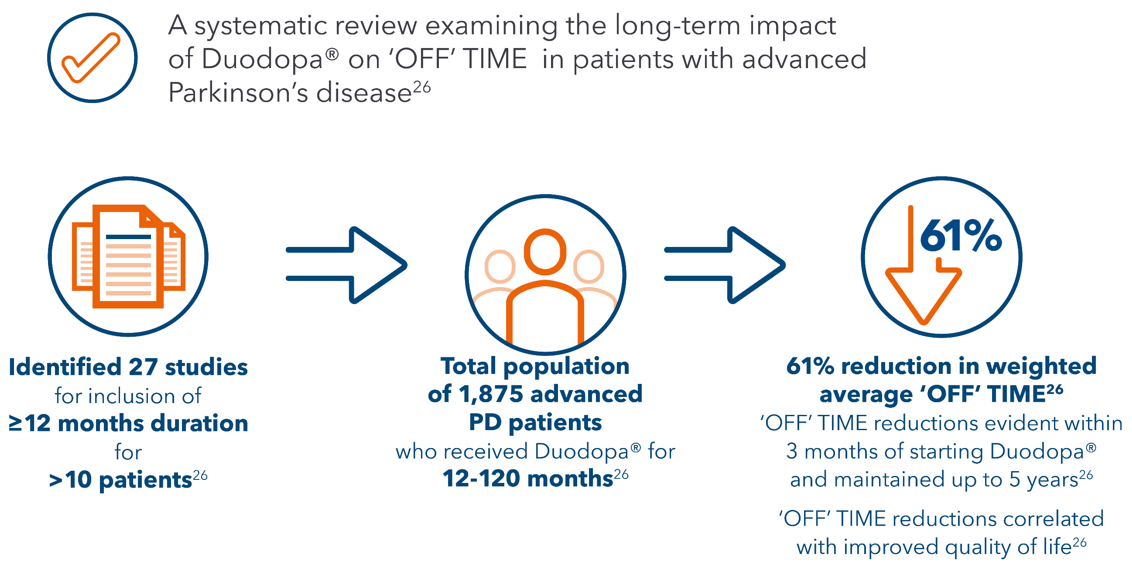
26. Antonini A, et al. Adv Ther. 2021 ;38:2854-90.
IE-DUOD-220036. Date of preparation: November 2022.